Uveitis in rheumatic diseases occurs due to a combination of autoimmune, genetic, and inflammatory mechanisms that disrupt the normal immune tolerance within the eye.
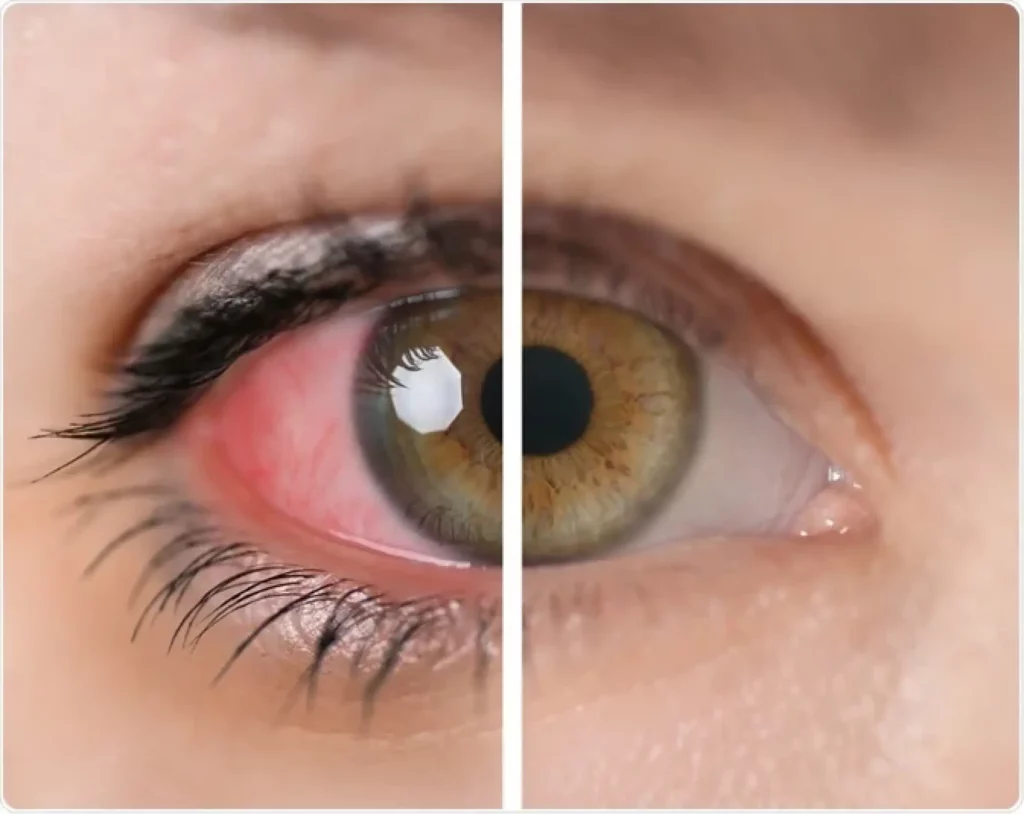
Uveitis is an inflammation of the uvea (the middle layer of the eye comprising the iris), ciliary body, and choroid. As a significant cause of visual impairment worldwide, uveitis demands attention, especially when linked to systemic conditions like rheumatic diseases. Understanding this connection is crucial for early diagnosis, effective treatment, and prevention of potential vision loss.
What is uveitis?
Uveitis refers to a group of inflammatory conditions affecting the uveal tract of the eye. Depending on the location of inflammation, uveitis is classified into:
– Anterior uveitis: involves the iris (iritis) or both the iris and ciliary body (iridocyclitis).
– Intermediate uveitis: affects the vitreous humor and peripheral retina.
– Posterior uveitis: involves the choroid (choroiditis), retina (retinitis), or both (chorioretinitis).
– Panuveitis: inflammation of all uveal components.
Symptoms may include eye redness, pain, blurred vision, floaters, and light sensitivity. Left untreated, uveitis can lead to serious complications, including glaucoma, cataracts, and even blindness.
Pathophysiology of uveitis
Uveitis in rheumatic diseases occurs due to a combination of autoimmune, genetic, and inflammatory mechanisms that disrupt the normal immune tolerance within the eye. Rheumatic diseases are systemic inflammatory conditions that primarily involve autoimmune dysfunction, where the body’s immune system mistakenly targets its own tissues. Several factors contribute to the development of uveitis in the context of rheumatic diseases.
Autoimmune mechanisms
Rheumatic diseases are characterized by an aberrant immune response, where immune cells recognize self-antigens as foreign, initiating an inflammatory response. In the case of uveitis, this process involves the ocular tissues. The eye is normally an immune-privileged site, meaning the immune system avoids mounting inflammatory responses to antigens in this area. However, in autoimmune conditions, mechanisms such as molecular mimicry can cause immune cells to target proteins in the eye that resemble those of pathogens or other foreign substances. This leads to inflammation of the uveal tract, which includes the iris, ciliary body, and choroid.
For example, in diseases like Ankylosing Spondylitis (AS), cross-reactivity between bacterial antigens and ocular proteins is thought to play a role in initiating acute anterior uveitis. Similarly, the systemic inflammatory process in conditions such as Reactive Arthritis or Psoriatic Arthritis can trigger immune responses that extend to ocular tissues.
Genetic predisposition
The genetic makeup of an individual plays a significant role in the susceptibility to both rheumatic diseases and uveitis. One of the most well-known genetic markers associated with uveitis in these diseases is HLA-B27, a major histocompatibility complex (MHC) class I molecule. HLA-B27 is strongly associated with seronegative spondyloarthropathies, including Ankylosing Spondylitis, Reactive Arthritis, and Psoriatic Arthritis.
HLA-B27 promotes the activation of immune cells, particularly T-cells, which play a significant role in mediating the immune response. In individuals with HLA-B27, there is a dysregulation of the immune system’s control over inflammation, increasing the risk of inappropriate immune activation in the eye. This can lead to recurrent episodes of uveitis, particularly in patients with spondyloarthropathies, where HLA-B27 is present in up to 50-60% of patients with uveitis.
Pro-inflammatory cytokines and immune cell activation
Pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-17 (IL-17) are central to the inflammatory processes seen in both rheumatic diseases and uveitis. These cytokines are critical in recruiting immune cells to sites of inflammation, including the eye. Elevated levels of these cytokines promote the infiltration of inflammatory cells, such as neutrophils, macrophages, and lymphocytes, into the uveal tissues, driving the inflammatory process that leads to uveitis.
In diseases like Behçet’s Disease and Juvenile Idiopathic Arthritis (JIA), for example, elevated cytokine levels, particularly TNF-α, are believed to mediate the chronic inflammation that leads to both systemic and ocular manifestations. TNF-α inhibitors, such as infliximab or adalimumab, are used to manage both systemic inflammation and uveitis, highlighting the importance of cytokines in the disease process.
Disruption of the blood-eye barrier
The eye is normally protected by the blood-eye barrier, which prevents immune cells and large molecules from entering the ocular environment. Inflammatory processes, such as those present in rheumatic diseases, can disrupt this barrier, allowing immune cells and inflammatory mediators to infiltrate the eye and contribute to uveitis.
In systemic diseases like sarcoidosis or granulomatosis with polyangiitis, granulomatous inflammation and systemic vasculitis can compromise the integrity of the blood-eye barrier. This allows immune cells, such as macrophages and T-cells, to invade the uveal tissues, leading to inflammation. Both anterior and posterior uveitis can occur in these conditions due to the widespread inflammatory activity.
Shared pathways of inflammation
In both rheumatic diseases and uveitis, there is an abnormal immune response involving T-helper cells (particularly Th1 and Th17 subsets), which secrete pro-inflammatory cytokines like TNF-α, IL-6, IL-17, and interferon-gamma (IFN-γ). These cytokines not only promote systemic inflammation but also directly contribute to inflammation within the eye, leading to breakdown of normal tissue structures and the onset of uveitis.
For example, in spondyloarthropathies (such as ankylosing spondylitis and psoriatic arthritis), Th17 cells, which are driven by IL-23, have a major role in both joint and ocular inflammation. This shared pathway explains why therapies that target IL-17 and IL-23, such as secukinumab, can be effective in treating both the articular and ocular manifestations of these diseases.
Molecular mimicry
The concept of molecular mimicry plays a significant role in the pathogenesis of uveitis in rheumatic diseases. Molecular mimicry occurs when immune cells, activated by antigens from infections or environmental stimuli, cross-react with similar self-antigens. This can cause the immune system to attack its own tissues, including the uveal structures in the eye.
For instance, in reactive arthritis, which often follows gastrointestinal or genitourinary infections (like Chlamydia trachomatis or Salmonella), immune cells activated against microbial antigens may cross-react with ocular tissues. This can lead to anterior uveitis, often in conjunction with other symptoms like conjunctivitis or keratitis. Such immune responses, although initially targeting external pathogens, become misdirected, affecting the eye.
Chronic systemic inflammation
Systemic inflammation is a hallmark of many rheumatic diseases, and chronic inflammation can predispose patients to secondary complications like uveitis. Persistent inflammation in the joints, skin, and gastrointestinal tract (as seen in psoriatic arthritis, inflammatory bowel disease, and juvenile idiopathic arthritis) can have distant effects, including ocular inflammation.
In diseases like juvenile idiopathic arthritis (JIA), particularly the oligoarticular subtype, chronic low-grade systemic inflammation can lead to insidious and often asymptomatic uveitis. Because of its silent nature, regular ophthalmologic monitoring is crucial in these patients to prevent irreversible vision loss.
Immune privilege breakdown
The eye is typically an immune-privileged site, meaning that it is protected from overactive immune responses to prevent damage to delicate structures. Immune privilege in the eye is maintained by the blood-ocular barrier, a physical and biochemical shield that limits the passage of immune cells and inflammatory mediators into the eye.
However, in the context of systemic autoimmune diseases, this immune privilege can be disrupted. Inflammatory cytokines such as TNF-α and IL-1 can cause breakdown of the blood-aqueous barrier and blood-retinal barrier, allowing inflammatory cells, such as T cells and macrophages, to infiltrate the uveal tissue. Once immune cells enter the eye, they release more cytokines and cause a cascade of inflammatory events, leading to tissue damage, fibrosis, and the clinical manifestations of uveitis.
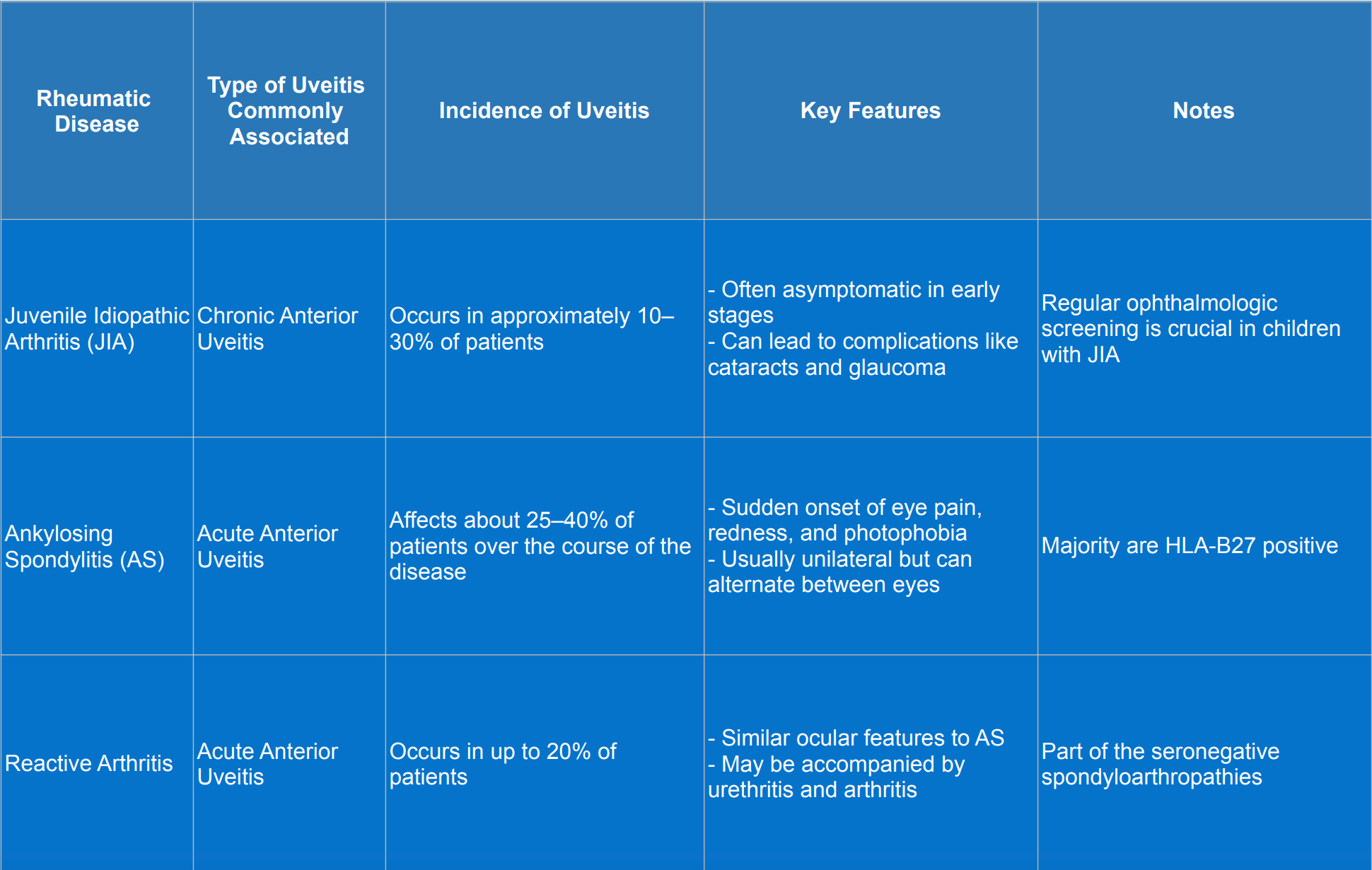
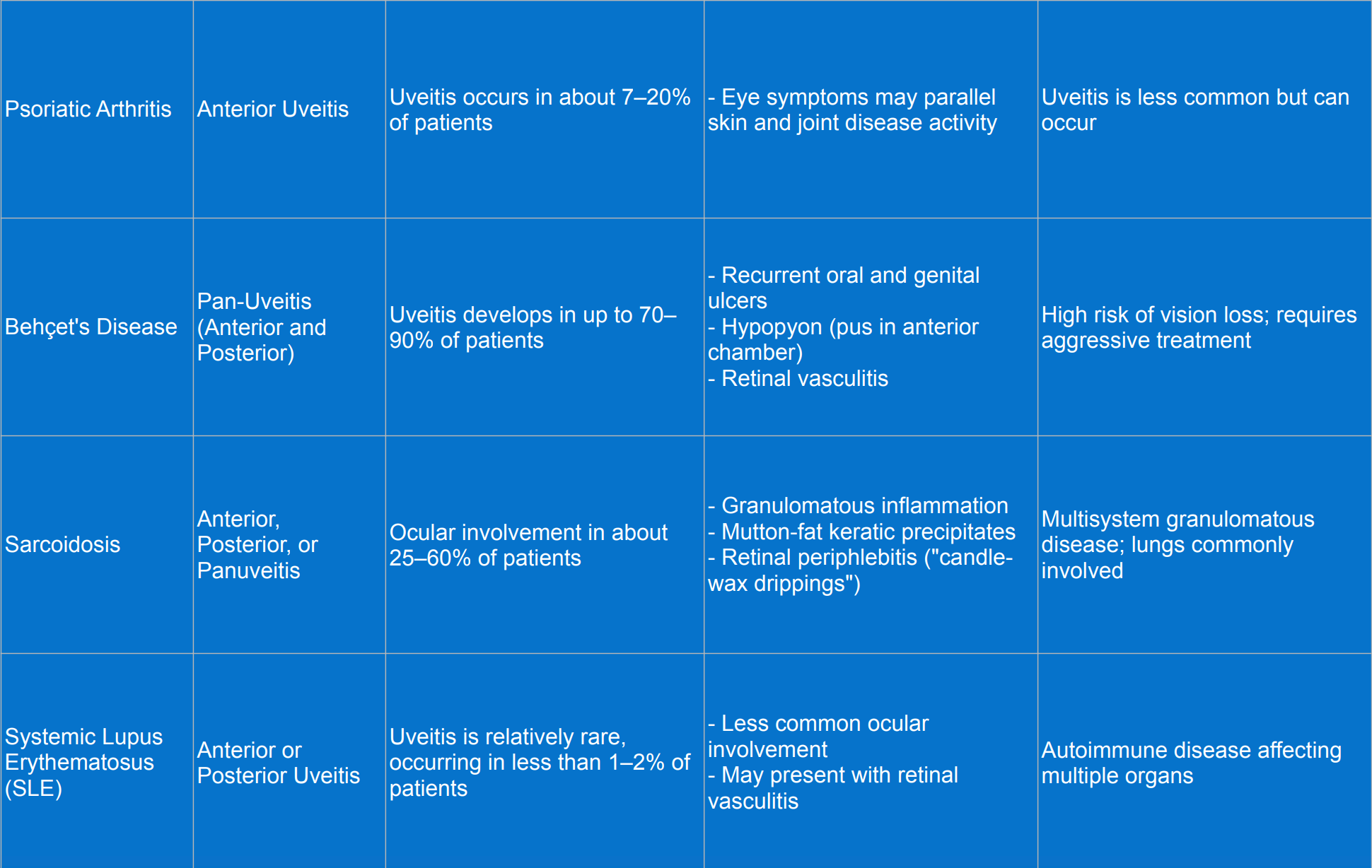
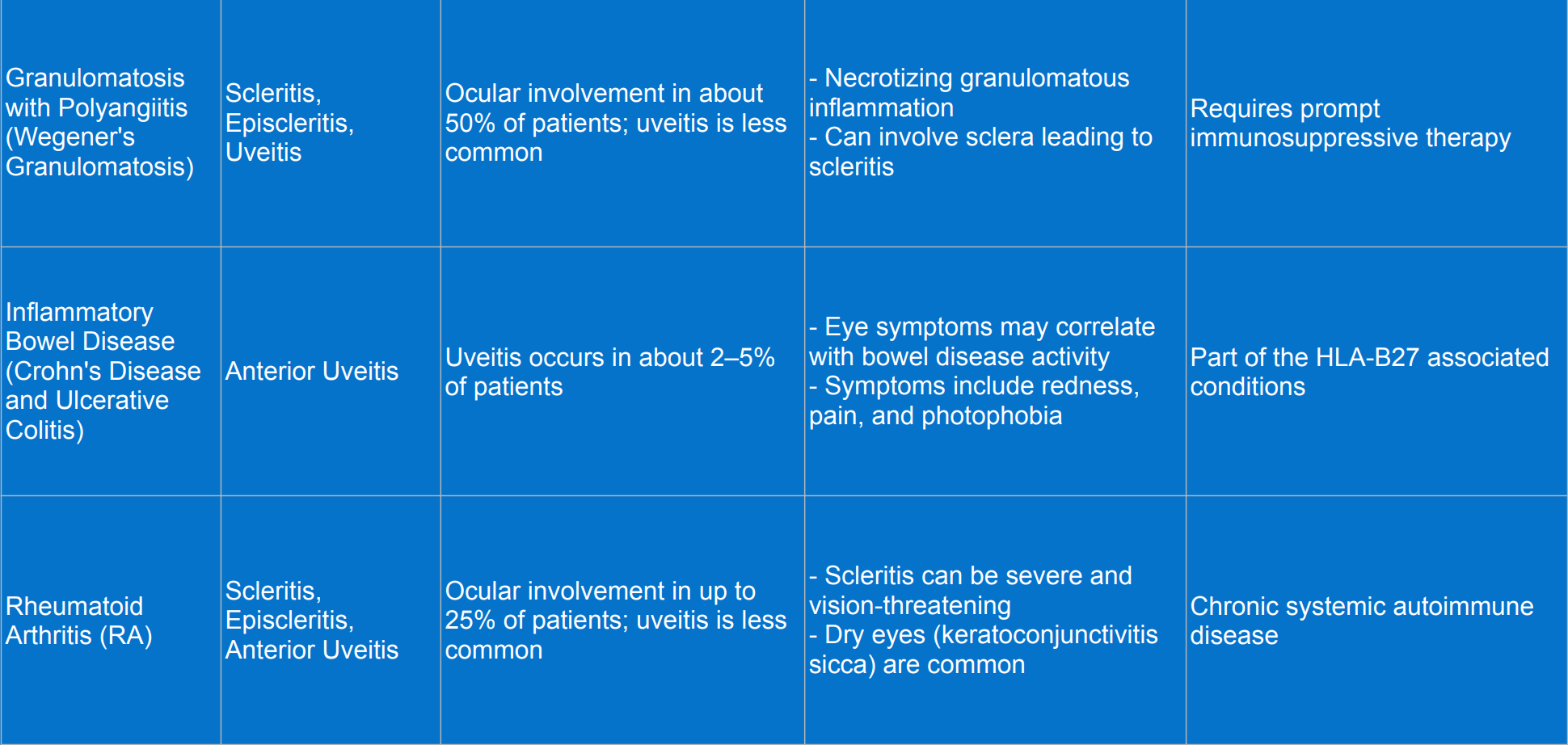
Rheumatic diseases associated with uveitis
Diagnosis of uveitis
Diagnosing uveitis in patients with rheumatic diseases requires a comprehensive and systematic approach to accurately identify the type, cause, and extent of inflammation. The process begins with a detailed medical history, paying close attention to ocular symptoms such as eye redness, pain, photophobia, blurred vision, and floaters. Understanding the onset, duration, and nature of these symptoms helps differentiate between various forms of uveitis. It is also important to inquire about any known rheumatic diseases or systemic symptoms like joint pain, skin rashes, or gastrointestinal issues that might suggest an underlying autoimmune condition.
The next step involves a comprehensive eye examination. Visual acuity is assessed to establish a baseline and detect any decrease in vision. A slit-lamp examination is crucial for inspecting the anterior segment of the eye, allowing observation of signs of inflammation such as cells and flare in the anterior chamber, keratic precipitates on the corneal endothelium, and posterior synechiae formation. For the posterior segment, a dilated fundoscopic examination is performed to evaluate the vitreous, retina, and optic nerve, looking for indicators like vitritis, retinal vasculitis, choroiditis, or optic disc edema.
Laboratory tests play a significant role in supporting the clinical findings. Specific blood tests are ordered to identify markers associated with uveitis and underlying rheumatic conditions. These may include HLA-B27 typing, antinuclear antibodies (ANA), rheumatoid factor (RF), anti-CCP antibodies, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and angiotensin-converting enzyme (ACE) levels. If an infectious cause is suspected, tests are conducted to rule out pathogens such as tuberculosis, syphilis, Lyme disease, herpes viruses, or toxoplasmosis.
Imaging studies provide valuable insights into the structural and functional changes within the eye. Optical coherence tomography (OCT) helps detect macular edema or other retinal abnormalities. Fluorescein angiography is used to assess the retinal vasculature for signs of leakage or non-perfusion, which can indicate retinal vasculitis. In cases where media opacities obstruct the view, such as dense cataracts or vitreous hemorrhage, ultrasound (B-scan ultrasonography) is utilized to evaluate the posterior segment for conditions like retinal detachment or choroidal masses.
Finally, interdisciplinary consultation is essential for a holistic approach to diagnosis and management. Close collaboration with rheumatologists helps confirm any underlying rheumatic diseases and coordinate systemic therapy, such as corticosteroids or immunosuppressive agents. If an infectious etiology is suspected, consulting infectious disease specialists can guide appropriate antimicrobial treatment. Depending on the patient’s presentation, referrals to other specialists such as pulmonologists, dermatologists, or gastroenterologists, may be necessary to address systemic involvement and ensure comprehensive care.
Treatment of uveitis
Treating uveitis in patients with rheumatic diseases requires a comprehensive strategy that targets both the ocular inflammation and the underlying systemic condition. The primary objectives are to control inflammation, prevent complications, and preserve vision.
Medical therapy
- Topical treatments: for anterior uveitis, topical corticosteroids such as prednisolone acetate eye drops are commonly prescribed to reduce intraocular inflammation. Cycloplegic agents like atropine or cyclopentolate may be used to relieve pain and prevent synechiae formation by dilating the pupil.
- Systemic corticosteroids: in cases of intermediate, posterior, or pan-uveitis, or when topical therapy is insufficient, systemic corticosteroids (oral or intravenous) are utilized to suppress inflammation throughout the eye.
Immunomodulatory therapy
- Immunosuppressive agents: when corticosteroids are contraindicated or long-term steroid use poses risks, immunosuppressive medications such as methotrexate, azathioprine, or mycophenolate mofetil are considered to control inflammation and reduce steroid dependency.
- Biologic agents: targeted biologic therapies, including tumor necrosis factor-alpha (TNF-α) inhibitors like adalimumab and infliximab, have shown effectiveness in treating uveitis associated with rheumatic diseases such as juvenile idiopathic arthritis and ankylosing spondylitis. Interleukin inhibitors may also be employed based on the specific rheumatic condition.
Management of underlying rheumatic disease
Addressing the systemic rheumatic condition is crucial for controlling ocular inflammation. Close collaboration with rheumatologists ensures that systemic disease activity is appropriately managed, which can lead to a decrease in uveitis episodes. Adjustments in systemic therapy, including disease-modifying antirheumatic drugs (DMARDs), may be necessary to achieve optimal control.
Monitoring and follow-up
Regular ophthalmologic assessments are essential to monitor the response to treatment, adjust therapies as needed, and detect potential side effects early. Follow-up evaluations typically include visual acuity tests, intraocular pressure measurements, and slit-lamp examinations. Educating patients about medication adherence and recognizing symptoms of recurrence or complications is important for successful long-term management.
Surgical interventions
In cases where medical therapy is insufficient or complications arise, surgical options may be necessary. Procedures such as vitrectomy can address persistent vitreous inflammation or complications like epiretinal membrane formation. Cataract surgery might be required for lens opacities resulting from chronic inflammation or prolonged corticosteroid use. Surgical intervention aims to restore visual function and reduce inflammatory load when other treatments have not been effective.
By implementing a tailored treatment plan that addresses both ocular and systemic aspects, healthcare providers can effectively manage uveitis in patients with rheumatic diseases, aiming to preserve vision and improve quality of life.
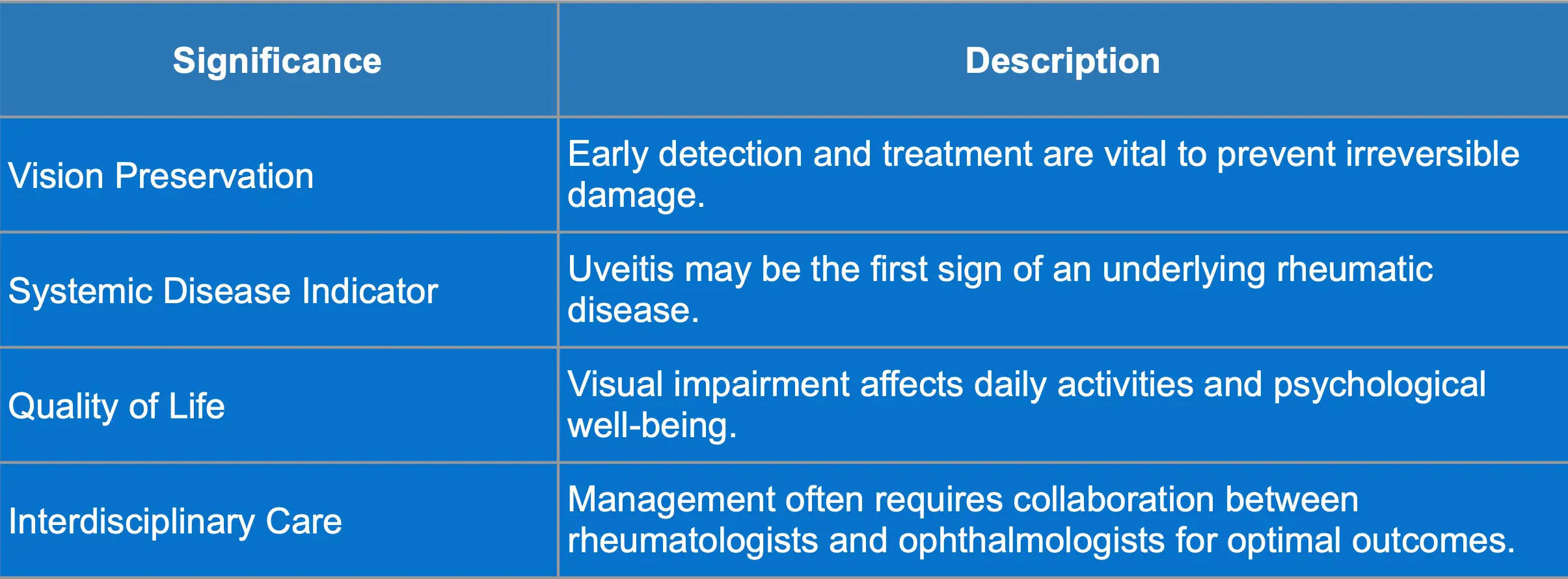
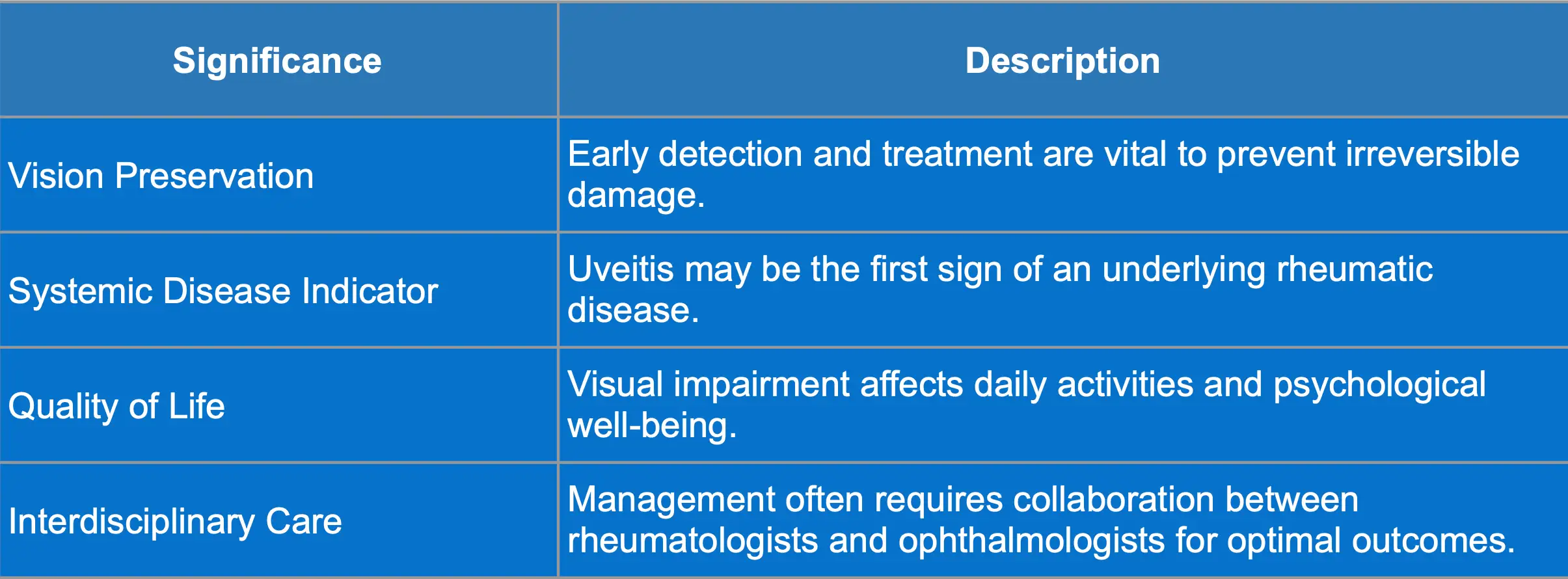
Complications and Significance of Uveitis
Possible complications and clinical significance of uveitis are summarized in the tables below:
Clinical case
Here is a clinical case to test your knowledge of uveitis in rheumatic disease. Read this case carefully and test your knowledge with the questions below:
Sarah is a 32-year-old elementary school teacher with a history of oligoarticular juvenile idiopathic arthritis (JIA) diagnosed at age 10, currently managed with methotrexate. She presents to the ophthalmology clinic complaining of blurred vision and mild discomfort in her right eye for the past week. She notes increased sensitivity to light (photophobia) and occasional floaters but denies severe pain or external redness. There is no history of recent trauma or infections, and her last rheumatology follow-up was six months ago, during which she was stable on her medication.
On ocular examination, her visual acuity is 20/25 in the right eye and 20/20 in the left eye. External examination shows no redness or discharge. Slit-lamp examination of the right eye reveals fine keratic precipitates on the corneal endothelium and mild anterior chamber cells (+1), indicative of inflammation. The posterior segment examination shows slight vitreous haze and cells in the vitreous humor of the right eye, while the left eye remains normal. Intraocular pressure is within normal limits in both eyes.
Additional workup includes positive antinuclear antibody (ANA) tests, negative HLA-B27, and mildly elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), suggesting systemic inflammation. Optical coherence tomography (OCT) does not detect any macular edema.
The diagnosis is chronic anterior uveitis in the right eye associated with JIA. The management plan involves initiating topical corticosteroids with prednisolone acetate eye drops on a tapering schedule to reduce intraocular inflammation. Cycloplegic agents like cyclopentolate eye drops are prescribed to relieve ciliary spasm and prevent posterior synechiae formation. A referral to her rheumatologist is made to reassess systemic disease activity and consider modifying her systemic therapy. Close monitoring with weekly ophthalmology visits is planned to assess her response to treatment and adjust medications as necessary.
Conclusion
Uveitis serves as an intersection between ocular health and rheumatic diseases. Through this clinical case, we see the importance of regular monitoring and interdisciplinary care in managing patients with rheumatic diseases. Recognizing the signs and initiating appropriate treatment promptly can significantly impact the patient’s quality of life and visual outcomes.
Study with MedBrane
If you’re keen to deepen your understanding of internal medicine, give the MedBrane app a try. It’s free and offers a great way to gauge what you already know through thorough exam simulations. Plus, you can learn as you go with in-depth explanations for every question.
Also you can follow us on Instagram @medbrane for more material for medical students and study tips!